Introduction
This is just one of the simple ways of identifying unknown compounds and separate mixtures. It is a separation technique that works based on the compound interactions as those compounds tend to move within a supporting medium. The compounds interact in two phases which are the mobile and stationary phase. The technique helps in analyzing, identifying, purifying and quantifying unknown separable mixtures. The mobile phase is either a liquid or gas which moves the solvent through the stationary phase during the process. The stationary phase is a liquid or solid component that is fixed in a place for the procedure.
Paper chromatography works majorly on capillary attractions. The capillary attraction which depends on adhesive and cohesive forces allows the mobile phase to move up the stationary phase due to created surface tension interaction from the forces. The major types are the paper chromatography, thin layer, gas chromatography, column chromatography, High performance liquid chromatography, paper chromatography, thin layer chromatography. There are several applications of paper chromatography and other main types of chromatography techniques. This technique is applicable in Pharmaceutical industries, hospitals, forensic science, environmental science and manufacturing plants.
This report describes the experiment conducted using paper chromatography to identify an unknown mixture. This will be done by comparing four known amino acids with the two unknown mixtures to identify the unknown mixtures. The experiment will also help master the technique and be able to analyze the movements made by both unknown mixtures and the known amino acids.
Materials
Gloves, goggles, lab coat, filter paper, toothpick, ninhydrin solution, mixtures to be identified and known amino acids.
Methods
The laboratory procedures entail different steps that eventually lead to identification of the unknown mixtures. This procedure is divided majorly into stationary phase preparation, mobile phase preparation and chromatograph development. For the stationary phase preparation, the required markings are made on the paper for identification and creation of baseline. The baseline marks are the 1.7cm from the shorter left edge and 1.0cm from the bottom of longer edge. Known amino acid symbols are mark on the paper. Spotting of the known four amino acids and two unknown mixtures are then done using separate toothpicks which will help prevent contamination.
Mobile phase preparation was done pouring 10ml of solvent mixture in a 400ml of Berzelius beaker while the chromatography development was done after the filter paper is already dried.
Data and Results
The solvent distance and spot distance were measured for all the points on the paper. The results is shown in the table below.
|
Amino Acids |
Solvent distance |
Spot distance |
Rf |
|
7.30 |
1.7 |
4.29 |
|
7.30 |
1.5 |
4.86 |
|
7.30 |
4.2 |
1.27 |
|
7.30 |
1.8 |
4.05 |
|
7.30 |
1.7 |
4.29 |
|
7.30 |
1.3 |
5.6 |
Picture showing the reactions, the spots of unknown mixtures and known amino acids
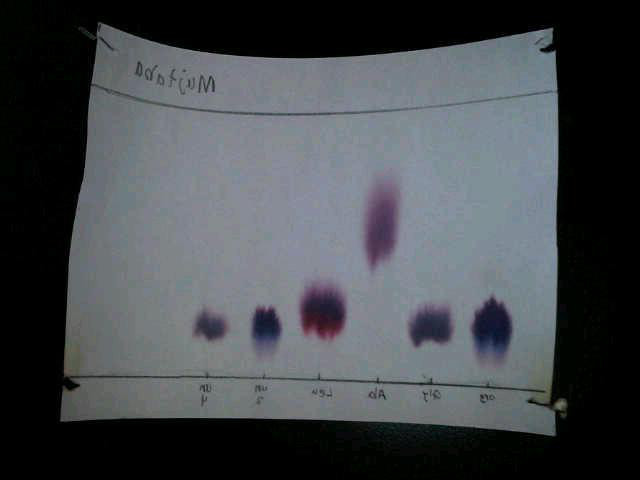
Discussion/Analysis
The collected data includes the solvent distance, and spot distance. The data was then used to calculate the presented R f results. The results clearly show the relationship of the unknown mixtures to the known amino acids. Analysing the colour of one of the mixture (the unknown mixture at spot 5) compared to the known amino acids especially the arginine, it was noted that they are more of the same purple colour. The findings of this experiment in terms of spots colour and Rf results shows that only one of the two unknowns is identified to be arginine. So it can be concluded that the first unknown mixture is an arginine amino acid or contain arginine amino acid as a component. The second unknown at spot 5 is not similar to any other known amino acids hence can’t be identified as same. It still remains unknown, but closer to glycine in identity. However, it can be considered to be glycine because of the difference in retention factor and slight difference in colour.
Conclusions
The findings of this paper chromatography experiment clearly shows the importance of paper chromatography in helping to identify unknown amino acids or analyze any other relevant mixtures that has properties of being separated by the paper. The theory of adhesion and cohesion plays an important part in the separation. The effects of the mobile and stationary phases are…